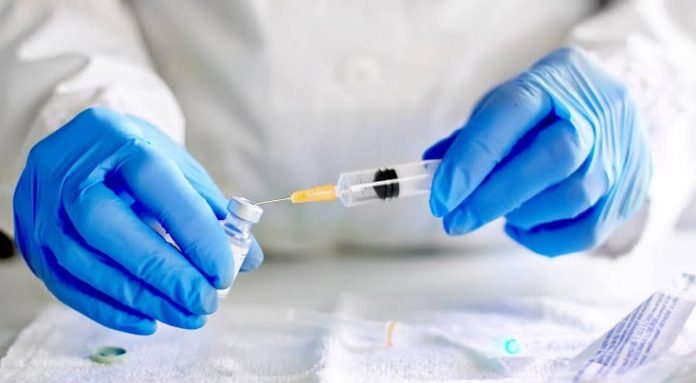
New Delhi (NVI): Bharat Biotech’s Covaxin, the indigenous Covid-19 vaccine, has been recommended for restricted use in emergency situation in public interest by Central Drugs Standard Control Organization (CDSCO) expert panel today.
This makes it the second vaccine after Serum-AstraZeneca’s Covishield to get marketing approval from the expert panel.
The recommendation, along with rollout modalities, will now be taken up by the Drug Controller General of India (DCGI) for a final decision on the matter.
Bharat Biotech had applied for emergency-use authorisation first on December 7. The expert panel at that time asked the firm to submit its safety and efficacy data from the ongoing phase 3 clinical trial for further consideration.
Biotech’s vaccine candidate is an inactivated one which are developed by inactivating (killing) the live micro-organisims that cause the disease. This destroys the pathogen’s ability to replicate, but keeps it intact so that the immune system can still recognise it and produce an immune response.
Restricted use approval is only granted if there is sufficient evidence to suggest the drug is both safe and effective.
-CHK